Antwort What is the formula for 1 to 10 dilution? Weitere Antworten – How do you calculate a 1 to 10 dilution

Commonly used dilutions are 1:10 or 1:2. Note that this is expressed as the ratio of the previous solution to the final volume of the dilution. For example, to make a 1:10 dilution, you add 1ml of your solution to 9ml of diluent for a final volume of 10ml.The formula for dilution factor (or DF for short) is as follows: DF = (final volume of cells + stain)/(initial volume of cells). For example, If you mix your sample 1:1 with AO/PI, you'll need to add 20 uL AO/PI to 20 uL cells, for a total of 40 uL.To complete a tenfold dilution, the ratio must be 1:10. The 1 represents the amount of sample added. The 10 represents the total size of the final sample. For example, a sample size of 1 ml is added to 9 ml of diluent to equal a total of 10 ml.
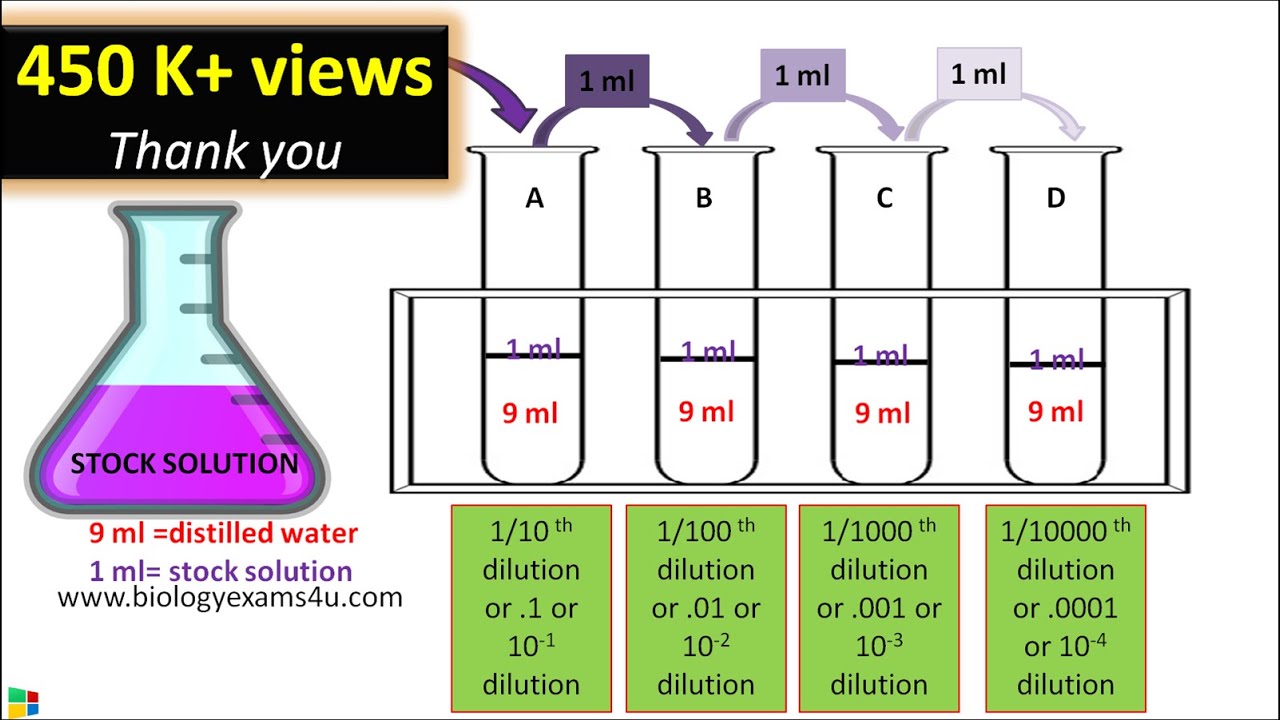
What is a 1 to 2 dilution : Another way of expressing this dilution is to state the ratio of sample-to-solvent (sample volume : added volume). For example, if 1 volume of sample is to be diluted by adding 2 volumes solvent, then we say that the sample should be “diluted 1:2” with solvent.
What percent is a 1 10 dilution
Dilution Charts and Conversion Tables
| Dilution Ratio | Ounces Per Gallon | Percent |
|---|---|---|
| 1:10 | 12.8 | 9.1% |
| 1:12 | 10.7 | 7.7% |
| 1:16 | 8 | 5.8% |
| 1:20 | 6.4 | 4.8% |
How many parts are needed for a 1 10 dilution : The dilution factor (DF) can be used alone or as the denominator of the fraction, for example, a DF of 10 means a 1:10 dilution, or 1 part solute + 9 parts diluent, for a total of 10 parts.
Dilution Formula
- Dilution refers to a drop in the pH of a chemical which can be a gas, vapour or solution.
- To prepare a fixed amount of dilute solution, we have a formula.
- C1V1 = C2V2.
- Where,
- V1 denotes the Volume of stock solution needed to make the new solution.
- V2 is the final volume of the solution.
First, determine the initial concentration before dilution (C1). Next, determine the dilution factor (D). In this case, the dilution factor is 10 for a 10-fold dilution. Next, use the formula C2 = C1 / D to calculate the final concentration after dilution (C2).
What is a 10x dilution example
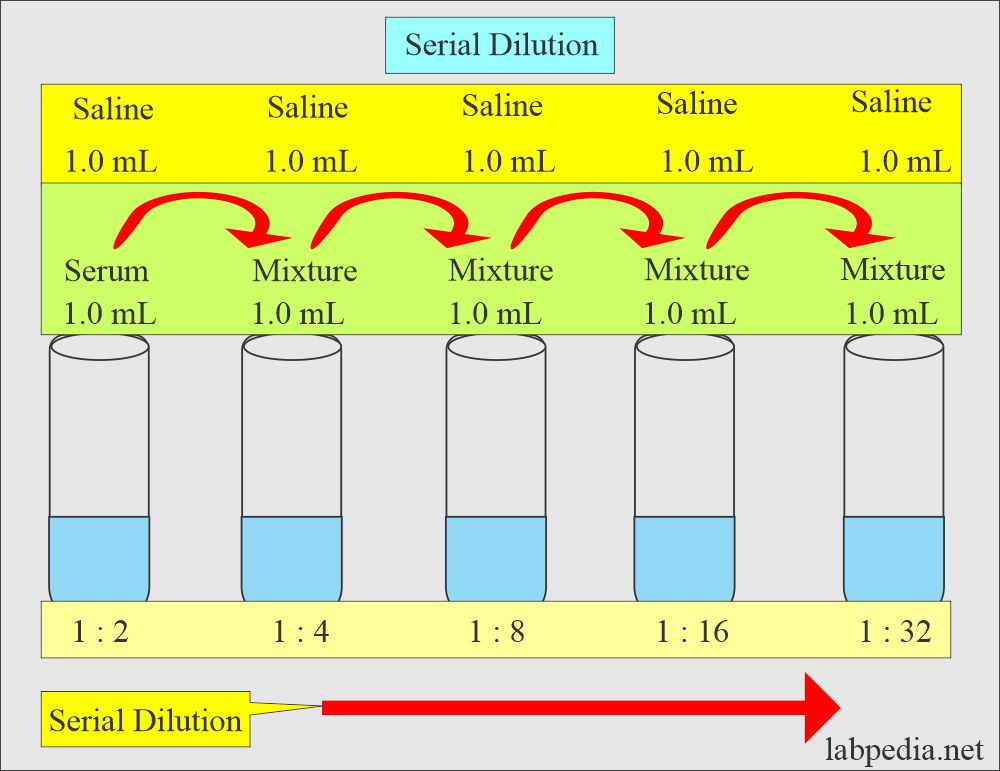
For example, let's say we have a 10x PBS stock and we want to make 1000mL of 1x PBS. To do this, we first need to divide 1000 mL into 10 parts, giving us 100 mL per part. Then, we will add 9 parts water and 1 part stock. This means we will add 900 mL water and 100 mL of the 10x stock to make 1x PBS.A 10x dilution is obtained by mixing 1 part of a sample with 9 parts of a diluent so that the new solution is 10 times (10x) less concentrated than the original solution. The 10x dilution can then be diluted by a factor of 10 again by mixing it with 9 more parts of the diluent.A 1 in 2 dilution means 1 part solute in 2 parts solution (a part can be volume ie; mL or mass ie; g). 1 mL solute + 1 mL of water = 2 mL solution at a dilution of 1 in 2. This means that the solute makes up 50% of the solution.
If you have a 1:3 dilution, i.e., a 1:3 dilution ratio, this means that you add 1 unit volume of solute (e.g., concentrate) to 3 unit volumes of the solvent (e.g., water), which will give a total of 4 units of volume.
How to calculate a ratio : Since ratios compare data between two numbers of the same kind, this means your formula would be A divided by B. For instance, if A equals 5 and B equals 10, then your ratio will be 5 divided by 10. Now, you're ready to solve the equation. Divide A by B to find a ratio. In this case, the answer is 0.5.
Is a 1 10 dilution same as a 10X dilution : Let's say you want to dilute a stock solution with water to make a working solution that is one tenth as concentrated. This could also be called a ten-fold dilution or a “10X” dilution, because the working solution will be ten times as dilute as the stock.
What is a 1 to 5 dilution
Answer: 1:5 dilution = 1/5 dilution = 1 part sample and 4 parts diluent in a total of 5 parts. If you need 10 ml, final volume, then you need 1/5 of 10 ml = 2 ml sample. To bring this 2 ml sample up to a total volume of 10 ml, you must add 10 ml – 2 ml = 8 ml diluent.
This tool calculates the volume of stock solution required to attain a desired concentration in a given volume. Stock concentration. M. Unit.Easiest way to calculate it is add the ratio together (e.g. 1:10 = 1+10 = 11) and divide the spray bottle capacity by that number. For a 500ml spray bottle, to mix a product 1:10 you would need to add 45ml of product (500/11) and 455ml of water ((500/11)*10).
How do you calculate a 10X dilution : A 10x dilution is obtained by mixing 1 part of a sample with 9 parts of a diluent so that the new solution is 10 times (10x) less concentrated than the original solution. The 10x dilution can then be diluted by a factor of 10 again by mixing it with 9 more parts of the diluent.


![csm_2405-bauerfeind-produktkategoriesseiten-bandagen-ellenbogenbandage-2560x1400_88-1_f91f66009c[1]](https://www.nakajimamegumi.com/wp-content/uploads/2024/06/csm_2405-bauerfeind-produktkategoriesseiten-bandagen-ellenbogenbandage-2560x1400_88-1_f91f66009c1-1024x521-65x65.jpg)
![Ischiasschmerzen[1]](https://www.nakajimamegumi.com/wp-content/uploads/2024/06/Ischiasschmerzen1-1024x640-65x65.jpg)
![csm_blogbeitrag_autoimmunerkrankung_d307ac8b72[1]](https://www.nakajimamegumi.com/wp-content/uploads/2024/06/csm_blogbeitrag_autoimmunerkrankung_d307ac8b721-1024x576-65x65.jpeg)